In search of more effective radiotherapy against cancer
By: Marisol Ortega Guerrero
Photos:
Health and Wellness

By: Marisol Ortega Guerrero
Photos:
A group of Colombian, French, and Canadian scientists hopes to bring good news to patients with thyroid cancer and gliomas (tumors that are developed in the brain and spinal cord). They are rigorously exploring how to optimize the use of radiation therapy to improve outcomes.
Thyroid cancer is the ninth most common cancer in the world and the seventh in Colombia, in both sexes and all age groups, according to the International Agency for Research on Cancer of the World Health Organization (WHO). Moreover, it is the fourth most frequent type of cancer among women. Gliomas, however, accounts for 15% of brain tumors.
State-of-the-art technologies, such as nanotechnology and metabolomics, are the tools that physicians are predominantly applying to understand why radiation therapy sometimes does not work and how to increase its effectiveness. The former looks at and employs materials with dimensions ranging from 1 to 100 nm (nanometers), whereas the latter looks at metabolites, which are the unique fingerprints left by all cellular processes.
The initiative emerged in 2018 in the Research Incubators on Biochemistry, Cancer and Radiobiology (SiBio), , at Universidad del Rosario, to support the construction and development of the translational research ecosystem of the School of Medicine and Health Sciences, that is, to promote interdisciplinary studies aimed at answering scientific questions that arise from clinical problems and proposing solutions.
“We chose gliomas because they respond to a model we had already been working on, especially glioblastoma, a cancer that is considerably resistant to radiotherapy. We found it a very interesting prototype to explore resistance markers. We also consider differentiated thyroid cancer because although it is radiosensitive, some patients develop resistance, and therefore, remain untreated,” explains Alejandro Oyono Ondo Méndez, a biochemistry and cellular and molecular biology specialist from the Biochemistry Unit of the Department of Biomedical Sciences at Universidad del Rosario.
Three years later, the idea became an interinstitutional and multidisciplinary project —which involved researchers from three universities in the country and other national and foreign institutions—called “Determination of metabolomic profiles of radiosensitivity and optimization of radiotherapy using nanoparticles in thyroid and glioma tumors: A translational approach".
Its aim is to establish the metabolomic profile of patients with thyroid or brain tumors that may be resistant to radiotherapy. Thus, researchers will be able to propose nanoplatforms or structures of drug delivery nanosystems in which various elements or molecules of biomedical interest can be included, resulting in a therapeutic tool for increasing tumor sensitivity to radiation, and thus, optimizing the radiotherapy use.
Professor Ondo is one of the researchers involved in this mission. Other members are Diana Rodríguez Burbano—a doctor in Chemistry and a specialist in the design and synthesis of nanomaterials for biomedical applications, from the Biomedical Engineering Program at Universidad del Rosario, in agreement with the Colombian School of Engineering— Julio Garavito, and Mónica Cala—a doctor in Chemistry, an expert in metabolomic analysis, and the director of the MetCore Metabolomics Core Facility at Universidad de los Andes.
The Colombian Geological Service, whose Nuclear Affairs Directorate regulates the use of ionizing radiation in Colombia, the Cancer Control Center,—an institution specialized in oncological radiotherapy—and the Hospital Universitario Mayor Mederi. are some of the institutions that have been interested in the study. Similarly, study groups from Universidad ECCI, Colombia, Université Côte d’Azur, France, and Concordia University, Canada, are participating.

Mónica Cala, director of the MetCore Metabolomics Core Facility at Universidad de los Andes.
The students at SiBio, who were the first to propose this initiative, conducted research on the best methods for prediction and decided that metabolomics was the best option for predicting radiation responses. The TIRO, research group from Côte d’Azur University suggested working with nanoparticles to improve the patient’s response to ionizing radiation.
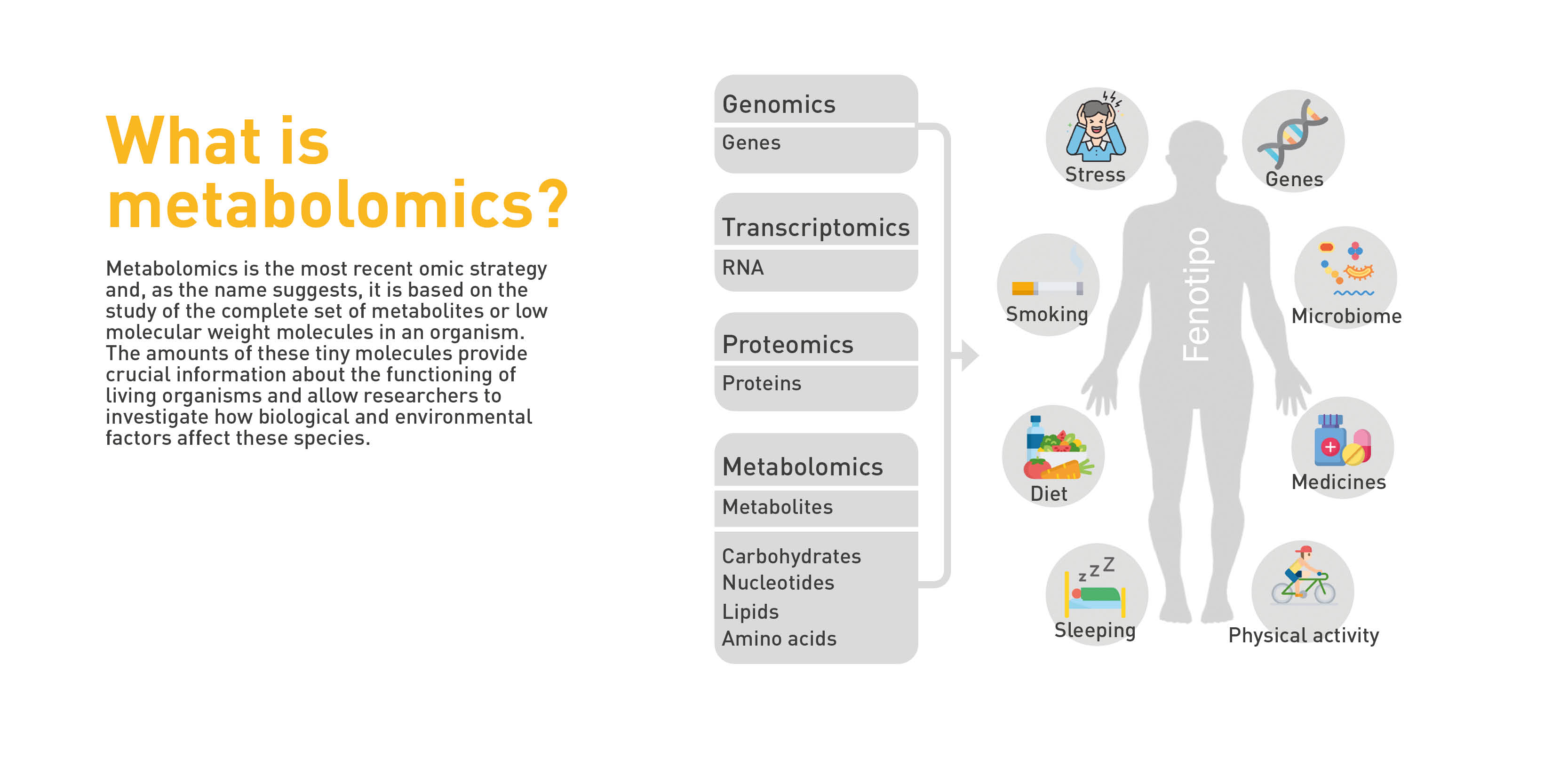
Metabolomics is the study of the set of substances called metabolites—amino acids, carbohydrates, and lipids—found in the biological processes of any organism. It allows the study of an organism’s phenotype, i.e., the set of visible characteristics that an individual presents because of the interaction between its genotype, genetic information, and the environment that surrounds it.
In thyroid cancer, metabolomics provides unique molecular information, such as a fingerprint left by cellular processes in their wake, which is the key in the diagnosis and/or prognosis of multiple tumor types. Knowing why some people respond to a treatment and others do not, for example, can help in early detection and improved therapeutic options.
As Dr. Cala points out, this technique enables us to detect metabolites and find the differences between them, elucidating the reason why they may be increased or decreased, when comparing blood or tissue samples from a healthy group to one with pathology. “This is critical in personalizing and stratifying patients to understand why some patients acquire the disease or respond to treatment and others do not.”
This information, as Ondo explains, helps in determining what cells do to protect themselves from radiation therapy and to identify possible therapeutic targets. In other words, establishing the metabolic processes or pathways that need to be impacted in cells to change the way they respond to radiation therapy. “If we know that in depth, we will be able to propose strategies to transform cells that develop a high level of resistance to radiotherapy into a more sensitive phenotype, and this would help us increase the efficacy of this therapy.”
With metabolomics, researchers seek to achieve customized medicine, not by devising a treatment for the tumor type in general, but for each individual patient. Their purpose is to see how well the tumor will respond to radiation to make the treatment as effective as possible
In terms of nanotechnology, the group aims to provide the most precise treatment possible by studying and manipulating extremely small sizes of matter, usually between 1 and 100 nm. This would be similar to using a color palette that allows for thousands of combinations.
“In the laboratory, we are designing and synthesizing nanoplatforms based on carbon and lanthanide ions to begin scaled studies on biological assays. In other words, we are conducting the first X-ray irradiation experiments on cells that were previously exposed to the nanoparticles,” says Professor Rodríguez, who is assisting in the design of the nanoplatform and its functionality, in association with researchers from Concordia University in Canada.
Such work is conducted with the support of physicists from the Colombian Geological Service, who simulate the structure of the nanoparticles being built in the project.
Their mission is to analyze how they interact with ionizing radiation, how many secondary electrons are produced, and under what conditions.
The study is now progressing well, despite the difficulties arising from the COVID-19 pandemic, which has limited the procurement of samples and other processes and has forced the experiments to be halted for several months.
“We are reactivating the cultures to perform studies on responses to radiotherapy for metabolomics. We aim to use samples from 25 thyroid tumors and 25 gliomas,” says Ondo.
Each sample of a tumor tissue is divided in two, one part for metabolomic studies and the other for evaluating radioresistance. The former is frozen in liquid nitrogen and the latter are subcultured in the laboratory and subsequently irradiated at the Cancer Control Center to analyze responses. By cross-referencing the information, defining metabolic expression and radioresistance profiles is possible.
Furthermore, the researchers perform physical simulations on the Geant4, platform —a software used to simulate the interaction of radiation with nanoparticles—that allows the establishment of the optimal environment (materials, size, concentration, and radiation dose) of possible nanoparticles that could be used as radiosensitizers.
Subsequently, the idea is to select samples of patients with radioresistance to test in vitro whether the effectiveness of radiotherapy increases when using nanoparticles and to analyze the metabolomic changes. This will provide new elements for the biological understanding of radiotherapy treatments, which will make it possible to develop personalized medicine for each patient in the future.

Alejandro Oyono Ondo Méndez, a researcher at the Biochemistry Unit of the Department of Biomedical Sciences, at Universidad del Rosario.

Diana Rodríguez Burbano from the Biomedical Engineering Program at Universidad del Rosario in agreement with the Colombian School of Engineering Julio Garavito.